
For Sol-Gel Abrasives, and Catalysts
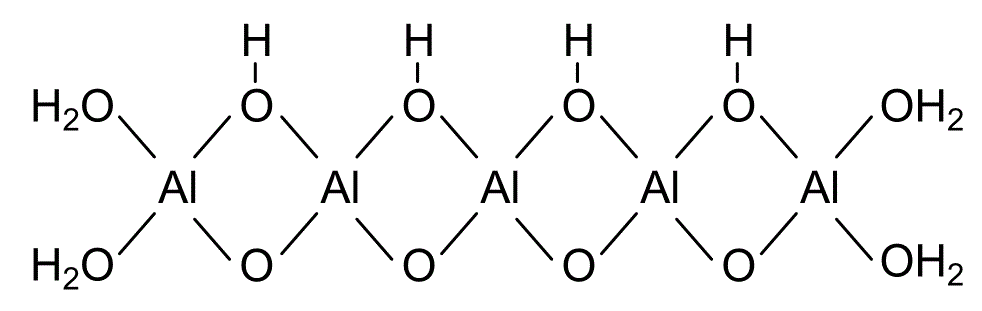
Pseudoboehmite or Boehmite (dispersible,Dispergierbare Böhmite) Sasol Pural is a nono alumina powder compound with the chemical composition AlO(OH).
It consists of finely crystalline boehmite. However, the water content is higher than in boehmite.
Pseudo-boehmite is white powder with high purity, odorless, tasteless,,low pile, large pore volume, high specific surface,good compatibility, excellent gelation and strong caking property. It can be easily decentralized and soluble in the acid and alkali solution. It can become highly active alumina after low-temperature roasting and dehydration.
In catalysts, Pseudoboehmite is used as binder for FCC catalysts and adsorbents,also a raw material for activated alumina.It is a binder of cracking catalyst for semi-synthetic rare-earth Y molecular sieve, a binder in aluminum silicate refractory fiber, a catalyst in making ethylene by alcohol dehydration and in making epoxy ethane.It also can be used as raw material for activated alumina and other aluminates.
In abrasives, these boehmites are nano-sized in the dispersed phase, exhibit a unique combination of purity, consistency and
dispersibility that make them excellent materials for use in colloidal applications, and in applications such as sol-gel ceramics,refractory materials, rheology control and surface frictionizing. The unique microstructure, composed of extremely uniform,sub-micron crystals, is designed to fracture conchoidally when stressed. The combination of the grit’s sharpness and structure allows for an aggressive cutting and long-lasting ceramic grain ideal for use in organic and vitrified bond-systems.
Technical Data Sheet
1. PseudoBoehmite: Small Porosity -General Na2O
Code | Al2O3 | Na2O | SiO2 | Fe2O3 | Porosity | BET | AcidDispersibility | Igloss | Density | Al2O3-3H2O | Particle Size (D10) | Particle Size (D50) | Particle Size (D90) |
GNa-1 | ≥66 | ≤0.30 | ≤0.25 | ≤0.02 | ≥0.35 | ≥250 | ≥95 | ≤33 | ≤0.80 | ≤3 | 1-3UM | 6-10UM | 16-35UM |
| Code | PseudoBoehmite,normal Na2O |
| Al2O3(%) | ≥66 |
| Na2O(%) | ≤0.30 |
| SiO2(%) | ≤0.25 |
| Fe2O3(%) | ≤0.02 |
| Porosity(ml/g) | ≥0.35 |
| BET(m2/g) | ≥250 |
| AcidDispersibility(%) | ≥95 |
| Igloss(%) | ≤33 |
| Density(g/cm3) | ≤0.80 |
| Al2O3-3H2O(%) | ≤3 |
| Particle Size(D10) | 1-3UM |
| Particle Size(D50) | 6-10UM |
| Particle Size(D90) | 16-35UM |
2. PseudoBoehmite: Small Porosity-Lower Na2O
Code | Al2O3 | Na2O | SiO2 | Fe2O3 | Porosity | BET | Acid Dispersibility | Igloss | Density | Al2O3-3H2O | Particle Size(D10) | Particle Size(D50) | Particle Size(D90) |
LNa-1 | ≥66 | ≤0.08 | ≤0.25 | ≤0.02 | ≥0.35 | ≥250 | ≥95 | ≤33 | ≤0.80 | ≤3 | 1-3UM | 6-10UM | 16-35UM |
| Code | PseudoBoehmite,lower Na2O |
| Al2O3(%) | ≥66 |
| Na2O(%) | ≤0.08 |
| SiO2(%) | ≤0.25 |
| Fe2O3(%) | ≤0.02 |
Porosity(ml/g) | ≥0.35 |
| BET(m2/g) | ≥250 |
| AcidDispersibility(%) | ≥95 |
| Igloss(%) | ≤33 |
| Density(g/cm3) | ≤0.80 |
| Al2O3-3H2O(%) | ≤3 |
| Particle Size(D10) | 1-3UM |
| Particle Size(D50) | 6-10UM |
| Particle Size(D90) | 16-35UM |

Pseudoboehmite
From Wikipedia, the free encyclopedia
Pseudoboehmite is an aluminium compound with the chemical composition AlO(OH). It consists of finely crystalline boehmite. However, the water content is higher than in boehmite.
History
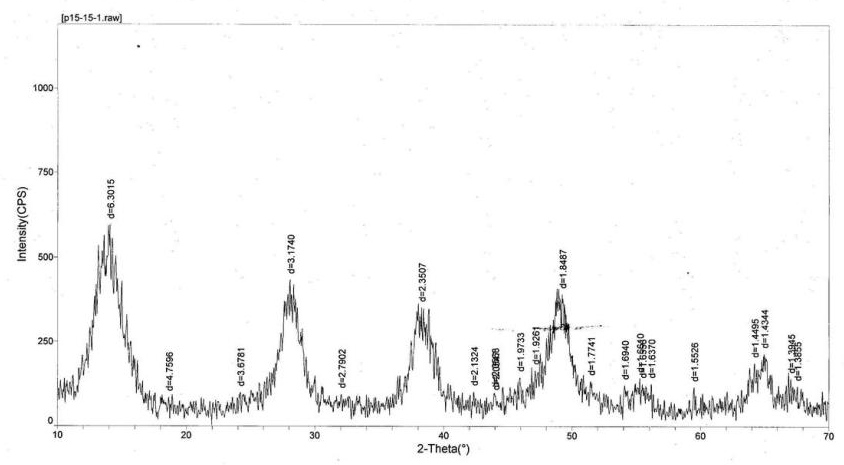
Calvet et al. coined the name pseudoboehmite in 1952 when they synthesized pure aluminium hydroxyde gel.[1][2] Its XRD pattern is similar to that of boehmite but the relative intensities of the peaks differ.
Morphology
Pseudoboehmite is essentially finely crystalline boehmite which consists of the same or similar octahedral layers in the xz plane but lacks three-dimensional order because of a restricted number of unit cells in y direction.[3] It consists of a significant number of crystallites which contain a single unit cell along y or single octahedral layers. It contains more water which is commonly intercalated between octahedral layers,normally randomly arranged, but sometimes regularly.
The water content consists of adsorbed and chemically bound water. The higher water content compared to boehmite can be explained by a smaller crystallite size.[4] While boehmite consists of relatively long AlOOH chains that have terminal H2O groups, the chains in pseudoboehmite are significantly shorter. This translates into a significantly higher specific water content due to the terminal water groups: